Step by step solution by experts to help you in doubt clearance & scoring excellent marks in exams. 0000003129 00000 n
b. d. Phosphorous pentoxide and calcium oxide are good drying agents, but they cannot be used to dry hydrogen chloride gas.  0000003617 00000 n
0000003617 00000 n
This domain has been purchased and parked by a customer of Loopia. 0000485662 00000 n
Give the balanced equation for the reaction. Jfvm(]CUqk"ev"d];ZlV9R-~Qf. 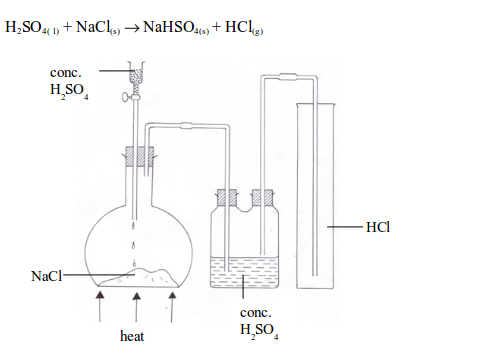 0000451526 00000 n
startxref
Create your website with Loopia Sitebuilder. 0000006126 00000 n
Exposure to 0.1 percent by volume hydrogen chloride gas in the atmosphere may cause death in a few minutes.
0000451526 00000 n
startxref
Create your website with Loopia Sitebuilder. 0000006126 00000 n
Exposure to 0.1 percent by volume hydrogen chloride gas in the atmosphere may cause death in a few minutes. 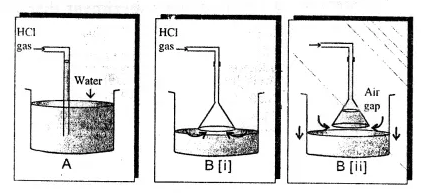 Answer the question that follow based on this reaction :
Answer the question that follow based on this reaction :
Name the drying agent not used for drying the gas. 0000422317 00000 n
:S!`{aXu^jUuw0c >nUZA e~r|(VsL+tX&VA80$. $KhJeq a.N6=. Hydrogen chloride may be formed by the direct combination of chlorine (Cl2) gas and hydrogen (H2) gas; the reaction is rapid at temperatures above 250 C (482 F). Get the revision notes in your mailbox! For this reason, hydrochloric acid is used extensively in the industrial processing of metals and in the concentration of some ores. 0000002979 00000 n
When sodium chloride is heated with concentrated sulphuric acid, the gas liberated is, Introduction to Three Dimensional Geometry. 0000408649 00000 n
%PDF-1.4
%
hbb8T0@ 9
Corrections?
0000011443 00000 n
endstream
endobj
238 0 obj
<>/Filter/FlateDecode/Index[164 44]/Length 21/Size 208/Type/XRef/W[1 1 1]>>stream
pitb h,p2)uFPj{ZfWZ
qf +- 0000002807 00000 n
Hydrogen chloride gas is prepared in the laboratory using concentrated sulphuric acid and sodium chloride. In aqueous solution the compound is extensively dissociated into a hydronium ion (H3O+) and chloride ion (Cl); in dilute solutions the dissociation is essentially complete. f. The funnel arrangement is done to dissolve HCl gas in water. These reactions occur readily only in the presence of moisture.
95+'I!8FR!yDPpjw-P/Pe|)~oE28cfx\tsL7(kZ9(j=<.3gV)0:Sv)?Iw trailer
A solution of the gas in water is called hydrochloric acid. Concentrated hydrochloric acid causes burns and inflammation of the skin. Please refer to the appropriate style manual or other sources if you have any questions. 0000010388 00000 n
Download our apps to start learning, Call us and we will answer all your questions about learning on Unacademy. Answer the question that follow based on this reaction :
Give the balanced chemical equation for the reaction with suitable conditions(s) if any. 239 0 obj
<>stream
A water solution containing 20.24 percent by weight hydrogen chloride boils at 110 C (230 F) without change in composition (azeotropic mixture). Hydrochloric acid is present in the digestive juices of the human stomach. Updates? d. Phosphorous pentoxide and calcium oxide are good drying agents, but they cannot be used to dry hydrogen chloride gasbecause they react with hydrogen chloride. Because of the corrosive nature of the acid, ceramic, glass, or sometimes tantalum apparatus is commonly used. hydrogen chloride (HCl), a compound of the elements hydrogen and chlorine, a gas at room temperature and pressure. Read more at loopia.com/loopiadns . Is magnesium hydride MgH2 an ionic compound class 12 chemistry JEE_Main, Write the equations for the preparation of 1iodobutane class 12 chemistry JEE_Main, The degree of hydrolysis for a salt of strong acid class 11 chemistry JEE_Main, The ratio of KpKcfor the reaction COg + dfrac12O2g class 11 chemistry JEE_Main, The reaction COg + 3H2g leftrightarrow CH4g + H2O is class 12 chemistry JEE_Main, Poly beta hydroxybutyrateco beta hydroxy valerate PHBV class 12 chemistry JEE_Main, Differentiate between the Western and the Eastern class 9 social science CBSE, CBSE Previous Year Question Paper for Class 10, CBSE Previous Year Question Paper for Class 12. Labelled Diagram for laboratory preparation of Hydrogen chloride is:a. Hydrochloric acid also enters into chemical reactions characteristic of the chloride ion, such as reactions with various inorganic and organic compounds in which hydrochloric acid is used as a chlorinating agent and reactions with metals and their oxides in which complex chloride-containing ions are formed (e.g., with platinum, [PtCl6]2, or with copper, [CuCl4]2).
Excessive secretion of the acid causes gastric ulcers, while a marked deficiency of it impairs the digestive process and is sometimes the primary cause of deficiency anemias. 0000423297 00000 n
0000410956 00000 n
Hydrogen chloride is a colourless gas of strong odour.
Use LoopiaWHOIS to view the domain holder's public information. Completely dry hydrogen chloride is very unreactive. 0000000956 00000 n
0000005216 00000 n
Protect your company name, brands and ideas as domains at one of the largest domain providers in Scandinavia. While every effort has been made to follow citation style rules, there may be some discrepancies. Why is this particular acid preferred to other acids? 0000004744 00000 n
Are you the owner of the domain and want to get started? Why? a.
The balanced equation for the reaction: \[\ce{NaCl + H2SO4 ->[<200C]NaHSO4 + HCl}\]. Therefore, it is not collected over water. Let QuizNexts Artificial Intelligence help you in precise revision. Click here to get PDF DOWNLOAD for all questions and answers of this Book - ICSE Class 10 CHEMISTRY. Take daily quizzes and stay on top! Aligned with 2021 board exam pattern. Concentrated H2SO4, b. It is also produced by the reaction of some chlorides (e.g., phosphorus trichloride, PCl3, or thionyl chloride, SOCl2) with water and as a by-product of the chlorination of many organic substances (e.g., methane or benzene). 0000006607 00000 n
Draw a labelled diagram for the laboratory preparation of hydrogen chloride gas and answer the following. 0
Hydrogen chloride is commonly prepared both on a laboratory and on an industrial scale by the reaction of a chloride, generally that of sodium (NaCl), with sulfuric acid (H2SO4). c. Name the drying agent used in drying hydrogen chloride gas. 0000470654 00000 n
It condenses at 85 C (121 F) and freezes at 114 C (173 F). 0000003701 00000 n
The reactions of hydrochloric acid are those of typical strong acids, such as: reactions with metals in which hydrogen gas is displaced, reactions with basic (metal) oxides and hydroxides that are neutralized with the formation of a metal chloride and water, and reactions with salts of weak acids in which the weak acid is displaced.
Let us know if you have suggestions to improve this article (requires login). 0000003815 00000 n
Hydrochloric acid is prepared by dissolving gaseous hydrogen chloride in water.
dRhSFasA/e`}=|M:!qVm]\a}u=u^I\M[P&FB/;U{VZC5V/o)z?+d45-wL
.l*]7w5 5Ne"5jCT6bs
{XZ@C@dJ7yikSuI}4.RJS*i\[bb@5?V14;@L('t
Yl*
7x`%IlJKMCZ1i
tLT?|]52*,3ON%E].X UrO/euT?0{iNFspLKl7$|6k;iBr.wt~4xFw4fV5.Dx`G}&~a&[jI(X !w.PIwcI4_]vt%q:C8hN\Hb7pQ.S*reB9/-xEO+*D Our editors will review what youve submitted and determine whether to revise the article. Select a Chapter from the menu to view the specific chapter. 0000011548 00000 n
Hydrochloric acid is usually marketed as a solution containing 2835 percent by weight hydrogen chloride, commonly known as concentrated hydrochloric acid. 0000018317 00000 n
G_9s 7
Hydrogen chloride gas is prepared in the laboratory using concentrated sulphuric acid and sodium chloride.
HUnFwx~8t@vTYWD#easR The reaction, represented by the equation H2 + Cl2 2HCl, is accompanied by evolution of heat and appears to be accelerated by moisture. endstream
endobj
209 0 obj
<>/Metadata 162 0 R/Pages 151 0 R/StructTreeRoot 164 0 R/Type/Catalog/ViewerPreferences<>>>
endobj
210 0 obj
<>/ExtGState<>/Font<>/ProcSet[/PDF/Text/ImageC/ImageI]/XObject<>>>/Rotate 0/TrimBox[0.0 0.0 612.0 792.0]/Type/Page>>
endobj
211 0 obj
[/ICCBased 228 0 R]
endobj
212 0 obj
[/Indexed 211 0 R 26 229 0 R]
endobj
213 0 obj
<>
endobj
214 0 obj
<>stream
f. What arrangement is done to dissolve HCl gas in the water? Hydrogen chloride gas is prepared in the laboratory using concentrated sulphuric acid and sodium chloride. 0000423609 00000 n
xref
Because of its great solubility, the gas fumes in moist air.
hVmPSW>|$7E7bLkkK`#!aaR?H; $B\kOlZq.3|g&7w}y79 ? `n0 0000012211 00000 n
Search available domains at loopia.com , With LoopiaDNS, you will be able to manage your domains in one single place in Loopia Customer zone. 0000408492 00000 n
0000018657 00000 n
https://revision-content-dev.s3.amazonaws.com/59c8b789-b558-4d11-a587-f92bc106f4a1.pdf, 2. (Qt`I0-R:E +@;m5bU/Fd{FG:J}#'H=o\5
Fus
Nnz6Dpt:>$H~{yaDR'm?_6x]C(P6Hza?W}op; c >zseHoXEn
Explanation of Laboratory Preparation of Hydrogen Chloride gas and Hydrochloric acid with diagram and reactions. General Introduction, Electronic Configuration and Occurence of Group 15 Elements (in Hindi), Oxidation States of Group 15 Elements with Explanation (in Hindi), Trends in Physical Properties of Group 15 Elements Part-1 (in Hindi), Trends in Physical Properties of Group 15 Elements Part-2 (in Hindi), Trends in Chemical Properties of Group 15 Elements Part-1 (Hydride) (in Hindi), Trends in Chemical Properties of Group 15 Elements Part-2 (Hydride) (in Hindi), Trends in chemical properties of group 15 elements part-3 (Oxides) (in Hindi), Trends in chemical properties of group 15 elements part-4 & Anomalous behaviour of Nitrogen (Hindi), Preparation, properties and Uses of Dinitrogen (in Hindi), Preparation of Ammonia (Laboratory preparation & Haber's process with explanation) (in Hindi), Properties of Ammonia with explanation (in Hindi), Preparation of Nitric acid ( Laboratory preparation & Ostwald's process) (in Hindi), Properties of Nitric acid with explanation (in Hindi), Brown Ring Test with explanation (in Hindi), Structure of oxides of Nitrogen with explanation Part-1 (in Hindi), Structure of oxides of Nitrogen with explanation Part-2 (in Hindi), Allotropic forms of Phosphorus (Structure, preparation and properties of all forms) (in Hindi), Preparation and properties of Phosphine (in Hindi), Halides of Phosphorus (Structure, Preparation and Properties) (in Hindi), Oxoacids of Phosphorous (structure, preparation and properties) Part-1 (in Hindi), Oxoacids of phosphorus (Structure, preparation and properties) Part-2 (in Hindi), Group 16 elements (Introduction, electronic configuration, oxidation state & occurence) (in Hindi), Trends in Physical properties of group 16 elements Part-1 (in Hindi), Trends in Physical properties of group 16 elements Part-2 (in Hindi), Trends in Chemical properties of group 16 elements Part-1 (Hydride) ( in Hindi), Trends in Chemical properties of group 16 elements Part-2 (Halide) (in Hindi), Trends in Chemical properties of group 16 elements Part-3 (Oxides) & Anomalous behaviour of oxygen, Preparation, properties and Uses of dioxygen (in Hindi), Ozone (structure, preparation, properties, uses and depletion of ozone layer) (in Hindi), Sulphur dioxide (Structure, preparation, properties and Uses) (in Hindi), Contact process (Industrial process) of manufacture of sulphuric acid (in Hindi), Physical properties, Chemical properties and uses of sulphuric acid (in Hindi), Structures of Oxoacids of Sulphur (in Hindi), General Introduction, Electronic configuration, Oxidation States and Occurence of group 17 elements, Trends in physical properties of group 17 elements Part-1 (in Hindi), Trends in physical properties of group 17 elements part-2 (in Hindi), Trends in Physical properties of group 17 elements Part-3 (in Hindi), Trends in Chemical properties of group 17 elements Part-1 (in Hindi), Trends in Chemical properties of group 17 elements Part-2 (in Hindi), Trends in Chemical properties of group 17 elements Part-3 ( in Hindi), Trends in Chemical properties of group 17 elements Part-4 (in Hindi), Anomalous Behaviour of Fluorine (in Hindi), Manufacture of Chlorine by Electrolytic process (in Hindi), Manufacture of Chlorine by Deacon's process (in Hindi), Physical Properties, Chemical Properties and Uses of Chlorine (in Hindi), Laboratory Preparation of Hydrogen Chloride gas and Hydrochloric acid (in Hindi), Physical Properties, Chemical Properties and Uses of Hydrogen Chloride (in Hindi), Group 18 (Intro, Electronic configuration, occurence & Trends in Physical properties) (in Hindi), Trends in Chemical properties of group 18 Elements Part-1 (in Hindi), Trends in Chemical properties of group 18 Elements Part-2 and Uses of group 18 elements (in Hindi), Unacademy is Indias largest online learning platform. 208 0 obj
<>
endobj
Gaseous hydrogen chloride reacts with active metals and their oxides, hydroxides, and carbonates to produce chlorides. Learn from our updated notes now. %%EOF
0000005520 00000 n
The gas is very soluble in water: at 20 C (68 F) water will dissolve 477 times its own volume of hydrogen chloride. Login to Loopia Customer zone and actualize your plan. Name the acid used. The following questions are pertaining to the laboratory preparation of hydrogen chloride gas:
How would you check whether or not the gas jar is filled with hydrogen chloride?
0000006848 00000 n
Q(YK '-bVp|9P,5s(D!A*Z Wj4K6,C@6.R,stVe2y|.f:5q)qR3n0PIw,BkQ azL2#O! Like this chapter so far? The latter type of reaction accounts for the ease of solution of certain metals and metallic compounds in hydrochloric acid although they are slowly dissolved in other acids of equal strength (e.g., sulfuric or nitric acid). Concept: Laboratory Preparation of Hydrogen Chloride Gas, Chapter 8 Study of Compounds - Hydrogen Chloride, Chapter 8: Study of Compounds - Hydrogen Chloride - Exercise 8 [Page 147], Maharashtra Board Question Bank with Solutions (Official), Mumbai University Engineering Study Material, CBSE Previous Year Question Paper With Solution for Class 12 Arts, CBSE Previous Year Question Paper With Solution for Class 12 Commerce, CBSE Previous Year Question Paper With Solution for Class 12 Science, CBSE Previous Year Question Paper With Solution for Class 10, Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Arts, Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Commerce, Maharashtra State Board Previous Year Question Paper With Solution for Class 12 Science, Maharashtra State Board Previous Year Question Paper With Solution for Class 10, CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 12 Arts, CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 12 Commerce, CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 12 Science, CISCE ICSE / ISC Board Previous Year Question Paper With Solution for Class 10. c. Thedrying agent used in drying hydrogen chloridegas is conc. Thus, hydrochloric acid is a strong acid. Get a Britannica Premium subscription and gain access to exclusive content. Omissions? SP)$AQ)s`IW>sOWy6c Q r|Hv[KX,a,XE%tb$R)v[$qU9>rJh{P3abv2.x;q(Oh0! This article was most recently revised and updated by, https://www.britannica.com/science/hydrogen-chloride, Agency for Toxic Substances and Disease Registry - Hydrogen Chloride. Anhydrous liquid hydrogen chloride is available, but because heavy and expensive containers are required to store it, the use of hydrogen chloride in this form is limited.
Laughter that comes from tickling is called gargalesis, and aside from primates the only animal known to experience it is the rat. 0000006238 00000 n
XH |OVq*m1)%mJ]Zk0h: dZ/C >dvYJ^d#L[HU8&\1@vw1j@ DtcC8?~bG7_SWftE..TfVRe,QuMA. Answer the question that follow based on this reaction :
Why is concentrated sulphuric acid used instead of concentrated nitric acid ? 208 32
sulphuric acid. Get Answer to any question, just click a photo and upload the photo and get the answer completely free, hello everyone today's question is HCL gas is prepared in laboratory using concentrated sulphuric acid and sodium chloride answer the questions that follow this on this reaction how is the gas collected the lab method used to prepare a real gases NaCl with concentrated H2 S o4 to give Sodium hydrogen sulphate with HCl gas now the method of collection of discus HCL gas is collected by downward delivery aur upward displacement of air which method is used to, collect HCL gas because this gas is 1.28 times heavier than air HCL gas is not collected over Water because this is highly soluble in water to upward displacement of air method is used to collect HCL gas thank you, Click here to get PDF DOWNLOAD for all questions and answers of this chapter - ICSE Class 10 SAMPLE PAPER 2020. With respect to the laboratory preparation of hydrochloric acid answer the following question :
Which method is used to collect hydrogen chloride gas ? (C>(t!0koO-mP@mgfXI^RBsIei*66Xvvwj*Y-xb
uw]9n 0000010808 00000 n
e. Why is the direct absorption of HCl gas in water not feasible? Properties and test of Hydrogen Chloride, Periodic Table, Periodic Properties and Variations of Properties, ICSE 10 Physics - Electrical Power and Household Circuits, ICSE 7 Chemistry - Acids, Bases and Salts, CBSE 10 Biology - Control and Coordination. 0000003654 00000 n
0000000016 00000 n
<]/Prev 1042250/XRefStm 2807>>
Our full-featured web hosting packages include everything you need to get started with your website, email, blog and online store. e. Hydrogen chloride gas is highly soluble in water.
- How To Use The Beurer Manicure Pedicure Set
- Sheraton Frankfurt Airport Address
- Gillette Fusion5 Razor Stand
- Address Plaques For Brick House
- Munich Tours From Airport
- Stone Fountains For Sale Near Me
- Hulk Toys Near Michigan
- Dust Right Separator Vs Dust Deputy
- Fuel Injection Fuel Cell
