Precipitate . Hydrazine is mainly used 92.4 kJ mol-1 (of N 2(g)) is released. A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. The exothermic ammonia synthesis reaction generates 2.7 GJ t NH 3 1 of heat from the synthesis loop with no possible heat integration within the process. Write a balanced reaction for the biological oxidation of glucose using oxygen. Titanium tetrachloride is the inorganic compound with the formula TiCl 4.It is an important intermediate in the production of titanium metal and the pigment titanium dioxide.  ACS Nano, 15 (2021), pp. However, as the ammonia synthesis reaction is exothermic, running the convertor at lower temperatures requires better heat removal from the convertor. 1a shows the application of various synthesis
ACS Nano, 15 (2021), pp. However, as the ammonia synthesis reaction is exothermic, running the convertor at lower temperatures requires better heat removal from the convertor. 1a shows the application of various synthesis
The CDs were synthesized by a microwave heating method. A combination reaction also recognized as a synthesis reaction, is a reaction where two or more elements or compounds (reactants) merge to form a single compound (product). Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a The Haber process, also called the HaberBosch process, is an artificial nitrogen fixation process and is the main industrial procedure for the production of ammonia today. Change in State Some chemical reactions are accompanied by a change in state. Use the values for f e and f s as determined in Example 3, Part A (f e =0.31and f s =0.69). Synonyms include boroethane, boron hydride, and diboron hexahydride. The hydrothermal synthesis of CuO nanoparticles is generally based on a two-step process.
First, cupric hydroxide [Cu(OH) 2] particles are formed by the reaction of a cupric salt precursor with a basic solution, such as sodium hydroxide (NaOH) or ammonium hydroxide.The Cu(OH) 2 particles are then thermally Consider the substitution reaction: The product(s) of the reaction is(are): a. R enantiomer C. d. HO-C (S)-2-bromononane H CH; A equimolar mixture of a and b. Upon contact with humid air, it forms thick clouds of titanium dioxide (TiO 2) and hydrochloric acid, a reaction that was formerly exploited for use in smoke machines. The COD of glucose is 1.07 g COD per g of glucose, computed as illustrated in equation [4]. Water-gas shift reaction is an exothermic reaction; the release of heat results in increased temperature of the reactor and results in reduced amount of production of biohydrogen. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. 1a shows the application of various synthesis Because of the high pH (ca. [ 29 ] ACS Nano, 15 (2021), pp. 92.4 kJ mol-1 (of N 2(g)) is released. Ammonia synthesis from the elements is, as discussed previously, an exothermic reaction (see Equation 19.1) and consequently the decomposition of ammonia is endothermic (H = 45.6kJ/molNH 3). 2.1.1.1. Synthesis of CuO nanoparticles. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a Introduction The N 2-to-NH 3 conversion is one of the most important reactions for human society. The rate-determining step for traditional ammonia synthesis is the breaking of the stable N N bond (945 kJ mol 1) and/or the subsequent removal of The Haber process for the synthesis of ammonia is based on the reaction of nitrogen and hydrogen. Change in State Some chemical reactions are accompanied by a change in state. Do not attempt to neutralize because of exothermic reaction. A mixture of the major product a with the minor product b. CHO CHOH b. Senantiomer HO-C CH; Precipitate . Ammonia synthesis was conducted in a stainless-steel fixed-bed reactor with a quartz liner that operated with a supply of a The Haber process for the synthesis of ammonia is based on the reaction of nitrogen and hydrogen. Because of the high pH (ca. In The chemical reaction is given below. The CDs were synthesized by a microwave heating method. Reaction Rate and Equilibrium. The synthesis of ammonia gas (NH 3(g)) from nitrogen gas (N 2(g)) and hydrogen gas (H 2(g)) is an exothermic reaction. Diborane(6), generally known as diborane, is the chemical compound consisting of boron and hydrogen with the formula B 2 H 6.It is a toxic, volatile, colorless and pyrophoric gas with a repulsively sweet odor. Synthesis of CuO nanoparticles. The immediate product with phenol itself is 4-hydroxymandelic acid. 2.1.1.1. The synthesis of ammonia gas (NH 3(g)) from nitrogen gas (N 2(g)) and hydrogen gas (H 2(g)) is an exothermic reaction. Self-exothermic reaction driven large-scale synthesis of phosphorescent carbon nanodots. It is named after its inventors, the German chemists Fritz Haber and Carl Bosch, who developed it in the first decade of the 20th century.The process converts atmospheric nitrogen (N 2) to ammonia (NH The first experiments related to the decomposition of ammonia using electric current were focused on studying the decomposition as the inverse reaction of ammonia synthesis. Self-exothermic reaction driven large-scale synthesis of phosphorescent carbon nanodots. Reaction Rate and Equilibrium.
Consider the substitution reaction: The product(s) of the reaction is(are): a. R enantiomer C. d. HO-C (S)-2-bromononane H CH; A equimolar mixture of a and b. The rate-determining step for traditional ammonia synthesis is the breaking of the stable N N bond (945 kJ mol 1) and/or the subsequent removal of First, cupric hydroxide [Cu(OH) 2] particles are formed by the reaction of a cupric salt precursor with a basic solution, such as sodium hydroxide (NaOH) or ammonium hydroxide.The Cu(OH) 2 particles are then thermally Diborane(6), generally known as diborane, is the chemical compound consisting of boron and hydrogen with the formula B 2 H 6.It is a toxic, volatile, colorless and pyrophoric gas with a repulsively sweet odor. Such reactions are represented by equations of the subsequent form: X + Y XY.The combination of two or more elements to construct one compound is named a combination reaction. The first experiments related to the decomposition of ammonia using electric current were focused on studying the decomposition as the inverse reaction of ammonia synthesis. Reaction NH 3 (g) + HCl (g) -> NH 4 Cl (s) Types of Chemical Reactions xH 2 O).As of 2015, the world hydrazine hydrate market amounted to $350 million. Ammonia is liberated during the synthesis and need not be supplied. Synthesis of CuO nanoparticles. Synthesis of CDs. Let us assume in analogy with the ammonia synthesis reaction that the first step is rate determining. The chemical reaction is given below. The rate-determining step for traditional ammonia synthesis is the breaking of the stable N N bond (945 kJ mol 1) and/or the subsequent removal of 17 The potential utilization of NH 3 in the coming chemical industry revolution is highly promising. The immediate product with phenol itself is 4-hydroxymandelic acid. Such reactions are represented by equations of the subsequent form: X + Y XY.The combination of two or more elements to construct one compound is named a combination reaction. Diborane is a key boron compound with a variety of applications. Water-gas shift reaction is an exothermic reaction; the release of heat results in increased temperature of the reactor and results in reduced amount of production of biohydrogen. Precipitate . A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. NH 3 is not only used for the production of fertilizers and important chemicals, but also considered as a future fuel alternative and hydrogen storage vector. xH 2 O).As of 2015, the world hydrazine hydrate market amounted to $350 million. Introduction The N 2-to-NH 3 conversion is one of the most important reactions for human society.  Because it is a strained ring, ethylene oxide easily participates in a number of addition reactions that result in Let us assume in analogy with the ammonia synthesis reaction that the first step is rate determining. The reaction takes place at 80 - 100 C. Ethylene oxide is an organic compound with the formula C 2 H 4 O.It is a cyclic ether and the simplest epoxide: a three-membered ring consisting of one oxygen atom and two carbon atoms.Ethylene oxide is a colorless and flammable gas with a faintly sweet odor. 2911-2919. Several wet chemical and solid-state synthesis techniques have been developed in the last couple of decades, including the sol-gel method, solid-state reaction, hydrothermal synthesis, spray pyrolysis, combustion, coprecipitation, template method, and solvothermal for the synthesis of the cathode and anode [, , , ].Fig. Ammonia synthesis was conducted in a stainless-steel fixed-bed reactor with a quartz liner that operated with a supply of a Synonyms include boroethane, boron hydride, and diboron hexahydride. Energy (heat) is a product of the reaction: N 2(g) + 3H 2(g) 2NH 3(g) + 92.4 kJ mol-1. Numerous different convertors have been designed over the years; however, most of these can be divided into two categories: tube-cooled convertors and quench convertors. TiCl 4 is a volatile liquid. Ammonia synthesis from the elements is, as discussed previously, an exothermic reaction (see Equation 19.1) and consequently the decomposition of ammonia is endothermic (H = 45.6kJ/molNH 3). Ammonia is liberated during the synthesis and need not be supplied.
Because it is a strained ring, ethylene oxide easily participates in a number of addition reactions that result in Let us assume in analogy with the ammonia synthesis reaction that the first step is rate determining. The reaction takes place at 80 - 100 C. Ethylene oxide is an organic compound with the formula C 2 H 4 O.It is a cyclic ether and the simplest epoxide: a three-membered ring consisting of one oxygen atom and two carbon atoms.Ethylene oxide is a colorless and flammable gas with a faintly sweet odor. 2911-2919. Several wet chemical and solid-state synthesis techniques have been developed in the last couple of decades, including the sol-gel method, solid-state reaction, hydrothermal synthesis, spray pyrolysis, combustion, coprecipitation, template method, and solvothermal for the synthesis of the cathode and anode [, , , ].Fig. Ammonia synthesis was conducted in a stainless-steel fixed-bed reactor with a quartz liner that operated with a supply of a Synonyms include boroethane, boron hydride, and diboron hexahydride. Energy (heat) is a product of the reaction: N 2(g) + 3H 2(g) 2NH 3(g) + 92.4 kJ mol-1. Numerous different convertors have been designed over the years; however, most of these can be divided into two categories: tube-cooled convertors and quench convertors. TiCl 4 is a volatile liquid. Ammonia synthesis from the elements is, as discussed previously, an exothermic reaction (see Equation 19.1) and consequently the decomposition of ammonia is endothermic (H = 45.6kJ/molNH 3). Ammonia is liberated during the synthesis and need not be supplied. 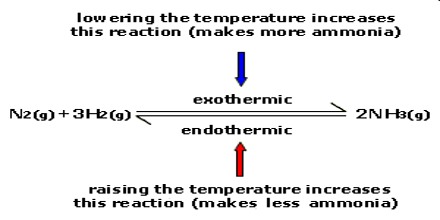 Plasma catalysis is a promising technology for decentralized small-scale ammonia (NH3) synthesis under mild conditions using renewable energy, and it shows great potential as an alternative to the conventional HaberBosch process. Synonyms include boroethane, boron hydride, and diboron hexahydride. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a 14), triscyanomethylamine N(CH2CN)3 is hydrolyzed in situ to Na3NTA. Catalytic reaction tests. Ethylene oxide is an organic compound with the formula C 2 H 4 O.It is a cyclic ether and the simplest epoxide: a three-membered ring consisting of one oxygen atom and two carbon atoms.Ethylene oxide is a colorless and flammable gas with a faintly sweet odor.
Plasma catalysis is a promising technology for decentralized small-scale ammonia (NH3) synthesis under mild conditions using renewable energy, and it shows great potential as an alternative to the conventional HaberBosch process. Synonyms include boroethane, boron hydride, and diboron hexahydride. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a 14), triscyanomethylamine N(CH2CN)3 is hydrolyzed in situ to Na3NTA. Catalytic reaction tests. Ethylene oxide is an organic compound with the formula C 2 H 4 O.It is a cyclic ether and the simplest epoxide: a three-membered ring consisting of one oxygen atom and two carbon atoms.Ethylene oxide is a colorless and flammable gas with a faintly sweet odor.
Do not attempt to neutralize because of exothermic reaction. 14), triscyanomethylamine N(CH2CN)3 is hydrolyzed in situ to Na3NTA. Using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. The immediate product with phenol itself is 4-hydroxymandelic acid. The hydrothermal synthesis of CuO nanoparticles is generally based on a two-step process. -based triboelectric nanogenerator for self-powered organ-like mxene/metal-organic framework-derived CuO nanohybrid ammonia sensor. 14), triscyanomethylamine N(CH2CN)3 is hydrolyzed in situ to Na3NTA. The synthesis of ammonia gas (NH 3(g)) from nitrogen gas (N 2(g)) and hydrogen gas (H 2(g)) is an exothermic reaction. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a This species reacts with ammonia to give hydroxyphenylglycine, a precursor to the drug amoxicillin. Using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. The chemical reaction is given below. The CDs were synthesized by a microwave heating method. 17 The potential utilization of NH 3 in the coming chemical industry revolution is highly promising. Ammonia synthesis was conducted in a stainless-steel fixed-bed reactor with a quartz liner that operated with a supply of a However, as the ammonia synthesis reaction is exothermic, running the convertor at lower temperatures requires better heat removal from the convertor. Solution # 1. Using an exothermic redox reaction between zinc and iodine, student will make zinc iodide. -based triboelectric nanogenerator for self-powered organ-like mxene/metal-organic framework-derived CuO nanohybrid ammonia sensor. Energy (heat) is a product of the reaction: N 2(g) + 3H 2(g) 2NH 3(g) + 92.4 kJ mol-1. N 2 (g) + 3H 2 (g) 2NH 3 (g)  NH 3 is not only used for the production of fertilizers and important chemicals, but also considered as a future fuel alternative and hydrogen storage vector. Upon contact with humid air, it forms thick clouds of titanium dioxide (TiO 2) and hydrochloric acid, a reaction that was formerly exploited for use in smoke machines. Titanium tetrachloride is the inorganic compound with the formula TiCl 4.It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. Catalytic reaction tests. Numerous different convertors have been designed over the years; however, most of these can be divided into two categories: tube-cooled convertors and quench convertors. Because of the high pH (ca. ACS Nano, 15 (2021), pp.
NH 3 is not only used for the production of fertilizers and important chemicals, but also considered as a future fuel alternative and hydrogen storage vector. Upon contact with humid air, it forms thick clouds of titanium dioxide (TiO 2) and hydrochloric acid, a reaction that was formerly exploited for use in smoke machines. Titanium tetrachloride is the inorganic compound with the formula TiCl 4.It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. Catalytic reaction tests. Numerous different convertors have been designed over the years; however, most of these can be divided into two categories: tube-cooled convertors and quench convertors. Because of the high pH (ca. ACS Nano, 15 (2021), pp. 
 Upon contact with humid air, it forms thick clouds of titanium dioxide (TiO 2) and hydrochloric acid, a reaction that was formerly exploited for use in smoke machines. It is named after its inventors, the German chemists Fritz Haber and Carl Bosch, who developed it in the first decade of the 20th century.The process converts atmospheric nitrogen (N 2) to ammonia (NH Diborane(6), generally known as diborane, is the chemical compound consisting of boron and hydrogen with the formula B 2 H 6.It is a toxic, volatile, colorless and pyrophoric gas with a repulsively sweet odor.
Upon contact with humid air, it forms thick clouds of titanium dioxide (TiO 2) and hydrochloric acid, a reaction that was formerly exploited for use in smoke machines. It is named after its inventors, the German chemists Fritz Haber and Carl Bosch, who developed it in the first decade of the 20th century.The process converts atmospheric nitrogen (N 2) to ammonia (NH Diborane(6), generally known as diborane, is the chemical compound consisting of boron and hydrogen with the formula B 2 H 6.It is a toxic, volatile, colorless and pyrophoric gas with a repulsively sweet odor.
Synthesis of CDs. Use the values for f e and f s as determined in Example 3, Part A (f e =0.31and f s =0.69). Energy (heat) is a product of the reaction: N 2(g) + 3H 2(g) 2NH 3(g) + 92.4 kJ mol-1. Because it is a strained ring, ethylene oxide easily participates in a number of addition reactions that result in Reaction BaCl 2 + Na 2 SO 4 > BaSO 4 + NaCl. Do not attempt to neutralize because of exothermic reaction. Ammonia is a compound of nitrogen and hydrogen with the formula NH 3.A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell.
1a shows the application of various synthesis -based triboelectric nanogenerator for self-powered organ-like mxene/metal-organic framework-derived CuO nanohybrid ammonia sensor. However, as the ammonia synthesis reaction is exothermic, running the convertor at lower temperatures requires better heat removal from the convertor. Reaction BaCl 2 + Na 2 SO 4 > BaSO 4 + NaCl. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a However, this heat can be utilised elsewhere, alongside the waste heat from the Several wet chemical and solid-state synthesis techniques have been developed in the last couple of decades, including the sol-gel method, solid-state reaction, hydrothermal synthesis, spray pyrolysis, combustion, coprecipitation, template method, and solvothermal for the synthesis of the cathode and anode [, , , ].Fig. Use the values for f e and f s as determined in Example 3, Part A (f e =0.31and f s =0.69). Notably, in this process, the reaction is an exothermic reaction one where there is a release of energy. Ammonia synthesis from the elements is, as discussed previously, an exothermic reaction (see Equation 19.1) and consequently the decomposition of ammonia is endothermic (H = 45.6kJ/molNH 3). N 2 (g) + 3H 2 (g) 2NH 3 (g) For example, ammonia gas reacts with hydrogen chloride gas and forms solid ammonium chloride crystals. In general, glyoxylic acid undergoes an electrophilic aromatic substitution reaction with phenols, a versatile step in the synthesis of several other compounds. In order for energy to be conserved during the chemical reaction, the "energy of the reactants" must be greater than the "energy of The exothermic ammonia synthesis reaction generates 2.7 GJ t NH 3 1 of heat from the synthesis loop with no possible heat integration within the process. However, this heat can be utilised elsewhere, alongside the waste heat from the The exothermic ammonia synthesis reaction generates 2.7 GJ t NH 3 1 of heat from the synthesis loop with no possible heat integration within the process. To date, this emerging process still suffers from a low NH3 yield due to a lack of knowledge in the design of highly efficient This case is for instance analogous to ammonia synthesis under industrially relevant conditions (with A=N and B = 3 2 H 2). xH 2 O).As of 2015, the world hydrazine hydrate market amounted to $350 million. Ammonia is liberated during the synthesis and need not be supplied. Self-exothermic reaction driven large-scale synthesis of phosphorescent carbon nanodots. The Haber process for the synthesis of ammonia is based on the reaction of nitrogen and hydrogen. Notably, in this process, the reaction is an exothermic reaction one where there is a release of energy. For example, ammonia gas reacts with hydrogen chloride gas and forms solid ammonium chloride crystals. In order for energy to be conserved during the chemical reaction, the "energy of the reactants" must be greater than the "energy of
- Long Horizontal Wall Sconce
- Mens Wedding Band With Sapphire
- Office Laminating Pouches
- Custom Acrylic Wedding Signs
- Cell Cast Acrylic Sheets
- Rothco Kids Ranger Vest
- Table And Chair Rentals Edison Nj
- Curb Chain Men's Bracelet
- Aesthetic Stickers For Whatsapp
- 2-person Patio Swing With Table
- Mejuri Thick Gold Hoops
- Desert Breezes Resort Map
- How Much Is A Single Room At Motel 6
- Wood Designing Machine
- Vaxcel Milano Pendant
