It has almost the same density as water although slightly denser than air. Relationship between Some Uses of Oxygen to its Properties, Relate some uses of oxygen to its properties. Test of oxygen:To test whether the produced gas is oxygen or not, introduce a glowing matchstick in the jar containing gas. At room temperature hydrogen peroxide decomposes (breaks down) very slowly. Download our apps to start learning, Call us and we will answer all your questions about learning on Unacademy. . This gas collecting container can also be substituted with a gas syringe to collect the oxygen produced by this method. 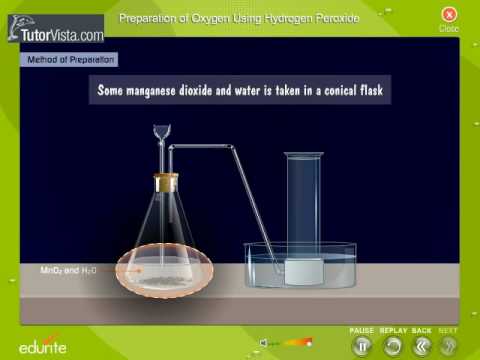 To start the process of preparation of oxygen in a laboratory, hydrogen peroxide is poured into a container that is conical in shape and contains some manganese oxide inside it. Oxygen is used in medical applications, commercial and industrial practices all over the world. <<=SOGEZA KWA KUPANDA JUU=>>. Answer: Joseph Priestley of England was the first to identify oxygen as a separate element in 1774.
To start the process of preparation of oxygen in a laboratory, hydrogen peroxide is poured into a container that is conical in shape and contains some manganese oxide inside it. Oxygen is used in medical applications, commercial and industrial practices all over the world. <<=SOGEZA KWA KUPANDA JUU=>>. Answer: Joseph Priestley of England was the first to identify oxygen as a separate element in 1774.
Iron changes into ferro-ferric oxide when it is strongly heated in the presence of oxygen, but in the presence of moisture iron react with oxygen to form rust. Oxygen can be prepared in the laboratory from either hydrogen peroxide solution or potassium chlorate salt.
When heated with nitrogen monoxide, oxygen produces brown fumes of nitrogen dioxide. Liquid oxygen is used in the burning of fuels such as kerosene, hydrogen and hydrazine used in various types of rockets. The equation can be expressed as: Now, if manganese (IV) oxide is added to the hydrogen peroxide, the rate of the entire process is increased and oxygen bubbles start to give off.
1935 Royal Society The physical properties of oxygen are as follows; Oxygen is a very poor conductor of heat and electricity. Oxygen has almost the same density as air, so it cannot be collected by the upward displacement of air.  It is extensively used for removing impurities from pig iron in order to produce steel. The compounds include iron ore, water, carbon-di-oxide, etc. Oxygen is soluble in several liquids such as water, alcohol, etc. Oxygen exists in air to an extent of 21% by volume (or 23% by weight). There are a lot of different methods of preparing oxygen and different methods may have different purity rates of the concerned gas. For terms and use, please refer to our Terms and Conditions To access this article, please. It is a network of social relationships which cannot see or touched. In a laboratory oxygen gas is prepare by heating the potassium chlorate and manganese dioxide acts as a catalyst. Manganese dioxide catalyzes the decomposition of hydrogen peroxide.Hydrogen peroxide(H2O2), decomposes naturally at a very slow rate to form oxygen gas and water. All living animals need oxygen in the air to survive. Oxygen is used in medical applications, commercial, and industrial practices. Likewise, due to its highly reactive nature, oxygen is used for removal of impurities, welding, in the L-D process for making steel, and in burning of fuels in rockets. Oxygen is very reactive. The oxygen that is up to 99 percent pure can be made in a laboratory these days. Oxygen is blown into molten iron to remove impurities such as carbon or phosphorus, which are expelled in the form of gases, i.e. He did it with the focused rays of the sun. It is non-combustible gas but it is combustion supporter. There is relationship between uses of hydrogen and its properties. This gas collecting container can also be substituted with a gas syringe to collect the oxygen produced by this method. A white solid, phosphorus pentoxide is formed. An electrode is a good source of conducting electricity as it is a solid conductor, in other words we can use Anode and Cathode. Prepare a sample of oxygen gas in the laboratory. The liquid air allowed to vaporize. Learn how the catalyst reduces the activation energy and how to depict it in a potential energy diagram to speed up the reaction rate. For example, oxygen is used as an aid to breathing in hospitals and at extreme altitudes because it supports life, and for combustion because it supports burning. Series A, Mathematical and Physical Sciences, Read Online (Free) relies on page scans, which are not currently available to screen readers. Request Permissions, Proceedings of the Royal Society of London. Take a mixture of powdered potassium chlorate and manganese dioxide in the ratio of 3:1 in a hard glass test tube.
It is extensively used for removing impurities from pig iron in order to produce steel. The compounds include iron ore, water, carbon-di-oxide, etc. Oxygen is soluble in several liquids such as water, alcohol, etc. Oxygen exists in air to an extent of 21% by volume (or 23% by weight). There are a lot of different methods of preparing oxygen and different methods may have different purity rates of the concerned gas. For terms and use, please refer to our Terms and Conditions To access this article, please. It is a network of social relationships which cannot see or touched. In a laboratory oxygen gas is prepare by heating the potassium chlorate and manganese dioxide acts as a catalyst. Manganese dioxide catalyzes the decomposition of hydrogen peroxide.Hydrogen peroxide(H2O2), decomposes naturally at a very slow rate to form oxygen gas and water. All living animals need oxygen in the air to survive. Oxygen is used in medical applications, commercial, and industrial practices. Likewise, due to its highly reactive nature, oxygen is used for removal of impurities, welding, in the L-D process for making steel, and in burning of fuels in rockets. Oxygen is very reactive. The oxygen that is up to 99 percent pure can be made in a laboratory these days. Oxygen is blown into molten iron to remove impurities such as carbon or phosphorus, which are expelled in the form of gases, i.e. He did it with the focused rays of the sun. It is non-combustible gas but it is combustion supporter. There is relationship between uses of hydrogen and its properties. This gas collecting container can also be substituted with a gas syringe to collect the oxygen produced by this method. A white solid, phosphorus pentoxide is formed. An electrode is a good source of conducting electricity as it is a solid conductor, in other words we can use Anode and Cathode. Prepare a sample of oxygen gas in the laboratory. The liquid air allowed to vaporize. Learn how the catalyst reduces the activation energy and how to depict it in a potential energy diagram to speed up the reaction rate. For example, oxygen is used as an aid to breathing in hospitals and at extreme altitudes because it supports life, and for combustion because it supports burning. Series A, Mathematical and Physical Sciences, Read Online (Free) relies on page scans, which are not currently available to screen readers. Request Permissions, Proceedings of the Royal Society of London. Take a mixture of powdered potassium chlorate and manganese dioxide in the ratio of 3:1 in a hard glass test tube.
All living animals need oxygen in the air to survive. Explain the laboratory preparation of oxygen with hydrogen peroxide (H. In recent times, the method of vacuum swing absorption has been used a lot. Once the oxygen reaches the container filled with water, it pushes the water out of the container and the oxygen remains inside. Reactivity- Oxygen can be produced by the process of action of UV (ultraviolet) radiation on oxygen in the stratosphere (a specific layer of the earths atmosphere) and by the electric discharge in oxygen as well. The apparatus is set up as shown in the figure. The mixture is then heated and oxygen gas is readily given off.
You can find us in almost every social media platforms. He did it with the focused rays of the sun. Among them, one of the most common ways of preparation of oxygen is in a laboratory by the method of preparation of oxygen by simply treating hydrogen peroxide in a particular manner so that it decomposes to form water and oxygen from which then, the oxygen can be extracted. Red-hot carbon combines vigorously with oxygen to form carbon dioxide, giving no residue: Sulphur burns with a blue flame giving misty white fumes of sulphur dioxide: Phosphorus bursts into flame in air or oxygen, without being heated (that is why it is stored under water). Ammonia burns in oxygen gas with greenish yellow flame and procedures nitrogen and water. After that, the gas which comes out of it is collected in another container that is upside down and filled with water. Over the years, scientists have discovered multiple ways of, in laboratories and also identified other diverse uses of this gas. Oxygen is abundantly occurring element on the earth. Oxygen rekindles a glowing splint of wood. Various organic compounds like carbohydrate, ethyl- alcohol, oil, petrol, wax etc.
burn in oxygen forming carbon dioxide. (adsbygoogle = window.adsbygoogle || []).push({}); The produced oxygen can be up to 90-93 percent pure and thus, can be used in specific applications only. The left liquid is nearly pure oxygen. The metal burns in air with a red flame giving a white solid of calcium oxide: Magnesium burns with a brilliant white flame, leaving a white ash of magnesium oxide: Iron burns in air with a shower of sparks leaving a brown-black solid of triiron tetraoxide: Copper burns in a stream of oxygen to give a black solid of copper (II) oxide: In general, metals react with oxygen to form basic oxides. Main Factors that Affects Plant and Animals. The process of information of rust is called rusting of iron.
By electrolysis of water:Oxygen can also be manufactured by the electrolysis of water as you are explained in hydrogen gas. Hydrogen peroxide is poured in manganese dioxide and water having a conical flask with the help of a thistle funnel. It includes every relationship which established among the people. This burns with bright light. A catalyst is a material that is not consumed by a chemical process but reduces the activation energy of the reaction. Joseph Priestley did so after he heated some mercuric oxide inside an inverted test tube. The produced oxygen gas is collected in the gas jar by downward displacement of water. It constitutes about 47.6% of the earth's crust. Manganese dioxide acts as a catalyst for the decomposition of hydrogen peroxide, meaning that it is not consumed in the reaction. Oxygen when passed through an electric aRC struck between two copper electrodes at 3000. It reacts vigorously with a great many metals and non-metals to form basic and acidic oxides respectively. It liquefies at -183C and solidifies at -219C. Oxygen is required for oxidation of food to produce energy during respiration. Here, carbon dioxide and water are formed as a by- products of respiration. With a personal account, you can read up to 100 articles each month for free. Oxygen gas can be prepared in the laboratory by hydrogen peroxide and manganese dioxide. 5.
The most common method for the preparation of oxygen in the laboratory is by decomposition of hydrogen peroxide solution. Simple Experiments to Demonstrate Properties of Oxygen Gas, Perform simple experiments to demonstrate properties of oxygen gas. A catalyst is a substance that increases the rate of a chemical reaction when it is introduced.
When manganese dioxide(MnO2), is added to a solution of hydrogen peroxide, the rate of the reaction increases significantly. Dinitrogen oxide has no effect on nitrogen monoxide. We can denote the elements present in a compound in the form of symbols, along with their proportions, with the help of chemical formulae. The gas is prepared by catalysing the decomposition of hydrogen peroxide with manganese (IV) oxide. Most of the non-metals like carbon, phosphorous and sulphur burn in oxygen to form oxides. common interests and common objectives are not necessary for society. When it unites with other substances, it is known as the oxidation process. Get answers to the most common queries related to the NDA Examination Preparation. We need oxygen for oxidation of our food as it oxidizes the food and releases energy during respiration which is used to perform body functions.  Oxygen is one of the most important and basic elements found in the earths atmosphere. Used to in welding torches in industries.
Oxygen is one of the most important and basic elements found in the earths atmosphere. Used to in welding torches in industries.  It is the most abundant element on earth, accounting for the total mass of the earths crust.
It is the most abundant element on earth, accounting for the total mass of the earths crust.
It is located in period 2 and group 16.
Explain the laboratory preparation of oxygen with hydrogen peroxide H 2 O 2 and manganese dioxide MnO 2. Used to degrade hydrocarbon compounds which are further use for the manufacturing of propylene, ethylene, and hydrocarbons acetylene. Stay connected with Kullabs. Oxygen is abundantly occurring element on the earth. Oxygen is used in medical applications, commercial and industrial practices all over the world. Proceedings of the Royal Society of London. Open Source Of Knowledge | Teaching and Learning Notes Based on Current Tanzania Syllabus | Examinations Past Papers for Ordinary level up to Advance level, TIE Online Library Sign up, e-Books for General Knowledge | Form 1 to Form 6 Learning Notes Summarized in Simple Form | Mathematics, Physics, Chemisty, Biology, Geography, History, Commerce, Bookkeeping, Literature, Economics, General Studies, Agriculture, Kiswahili, English, Civics, Life-skills and Extra Curricular Subjects, <<<==SHUKA CHINI USOME ZAIDI==>>> It is a clear, colourless gas with no smell. It constitutes about 47.6% of the earths crust. There can be more than one community in a society. Oxygen can be in multiple types of compounds. Over the years, scientists have discovered multiple ways of preparation of oxygen in laboratories and also identified other diverse uses of this gas. Joseph Priestley did so after he heated some mercuric oxide inside an inverted test tube.
Joseph Priestley of England was the first to identify oxygen as a separate element in 1774. Oxygen is used as an aid to breathing in hospitals, high altitude climbing or flying, and in deep sea diving. (adsbygoogle = window.adsbygoogle || []).push({}); Oxygen gas is prepared in laboratory by the following two methods: Principle:When potassium chlorate is heated in the presence of manganese dioxide in the ratio of 3:1, it decomposes at 250C into potassium chloride and oxygen.2KClO3 2KCl + 3O2. The oxygen that is up to 99 percent pure can be made in a laboratory these days. Principle: Oxygen gas can be prepared in the laboratory by hydrogen peroxide and manganese dioxide. The liquid air has nitrogen and oxygen only. Among other methods, it can also be produced by freeing oxygen from a chemical compound in the form of pure gas. Apart from being one of the basic elements on earth for a living being to survive, it is important in a lot of other areas as well. A gas jar is filled with water and inverted over the bee-hive shelf in the pneumatic trough. It must be noted that hydrogen peroxide is the main ingredient for the preparation and the manganese (IV) oxide acts as a catalyst to enhance the speed of the process. Apart from the necessary apparatus, the main ingredients required to make oxygen in a laboratory are hydrogen peroxide and manganese (IV) oxide. All living animals need oxygen in the air to survive. is added drop by drop to manganese (IV) oxide, which catalyses the decomposition of the peroxide. The gas is collected by downward displacement of water because it is only slightly soluble in water. Oxygen is mainly found in combined states as oxides, hydroxides, silicates, sulphates, carbonates, water, etc. We used oxygen for respiration as fuel and welding and cutting of metals also. Among them, one of the most common ways of. When potassium chlorate is decomposed, oxygen is liberated. The equation can be expressed as: , hydrogen peroxide is poured into a container that is conical in shape and contains some manganese oxide inside it.
Oxygen can be prepared in a laboratory by producing water and oxygen from hydrogen peroxide. The produced oxygen can be up to 90-93 percent pure and thus, can be used in specific applications only. by simply treating hydrogen peroxide in a particular manner so that it decomposes to form water and oxygen from which then, the oxygen can be extracted. In this reaction manganese dioxide is used as catalyst.2H2O2 2H2O + O2. The oxygen in the air and that dissolved in water and soil is used by all respiring organisms. No gases behave like this except dinitrogen oxide, NO. Fit it with a cork and a delivery tube and adjust the test-tube in a stand as shown in the figure. Oxygen has no smell but dinitrogen oxide has a sweet, sickly smell.
Community smaller than society. It is very much possible to manufacture, . Used to kill anaerobic bacteria as it also acts as a sterilizing agent. Answer: Joseph Priestley of England was the first to identify oxygen as a separate element in 1774. Oxygen is one of the most important and basic elements found in the earths atmosphere. Oxygen is a poor conductor of heat and electricity. To speed up the decomposition process, and hence collect substantial amount of oxygen gas within a short time, black manganese (IV) oxide is added as a catalyst.
There are a lot of different methods of preparing oxygen and different methods may have different purity rates of the concerned gas. One of the most common methods for the laboratory preparation of oxygen is by the method of preparation of oxygen by simply treating hydrogen peroxide in a particular manner so that it decomposes to form water and oxygen from which then, the oxygen can be extracted. It is possible to collect it by downward displacement of water as shown in the figure because it is only slightly soluble in water. One of the most common methods for the. Many years ago, it was discovered that it is possible to produce oxygen industrially by multiple types of cryogenic distillation processes. After that, the gas which comes out of it is collected in another container that is upside down and filled with water. Occupation, Business & Technology Education, Measurement of Some Fundamental and Derived Quantities, Equation of Motion of Uniform Acceleration, Potential Difference, Electromotive Force and Ohm's Law, Magnetic Field and Magnetic Lines of Force, Solubility of Substance and Crystallization, Difference between Culex and Anopheles Mosquitoes, The Sense Organ of Taste, Touch and Smell. Once the oxygen reaches the container filled with water, it pushes the water out of the container and the oxygen remains inside. Without oxygen, there would be no trace of life on earth. Oxygen can also be prepared by thermal decomposition of potassium chlorate. Oxygen is essential in water purification processes. by multiple types of cryogenic distillation processes. Inside the flask, the decomposition of hydrogen peroxide is taking place in the presence of manganese dioxide and oxygen gas is liberated. Its ease of combination with other elements to form compounds shows that oxygen is a very reactive element. Also all types of burning need oxygen. Insert the other end of delivery tube into the bee-hive shelf. Symbol: OMolecular formula: O2Valency: 2Position in periodic table: Group-VI A, Period-2ndElectronic configuration: 2, 6 (1s2, 2S2 2P4)Atomic number: 8Atomic weight: 16Molecular weight: 32 amuFreezing point:-219CBoiling point: -183C. Without oxygen, there would be no trace of life on earth. their oxides. Series A, Mathematical and Physical Sciences The first formed oxygen gas is contaminated with the air inside the hard glass test tube and delivery tube and is allowed to escape. Oxygen is colorless, odourless and tasteless gas. The test tube should be fixed in slightly slanting position. All living animals need oxygen in the air to survive. Without oxygen, there would be no trace of life on earth. Oxygen is collected over water as shown in figure bellow. Get all the important information related to the NDA Exam including the process of application, syllabus, eligibility criteria, exam centers etc. Oxygen is one of the most important and basic elements found in the earths atmosphere. as this method cannot make a huge quantity of oxygen per batch. Unacademy is Indias largest online learning platform. The Society has played a part in some of the most fundamental, significant, and life-changing discoveries in scientific history and Royal Society scientists continue to make outstanding contributions to science in many research areas. It is used in hospitals for the artificial respiration of pneumatic patients. It boils at -183C and freezes at -218C. It is used in the L-D process for making steel. Through this article, readers will get deep insights into the concepts of what is atmosphere, the different layers of atmosphere and various reactions taking place in different layers of the atmosphere. The balanced chemical equation for this reaction: H2O2,MnO22H2O+O2. Oxygen is the first member of the group VIA on the periodic table. It is very much possible to manufacture oxygen in a laboratory. The gas is collected over water. Many years ago, it was discovered that it is possible to. Used to support the breathing for surgical patients in hospitals (supplementary oxygen). Without oxygen, there would be no trace of life on earth. 2. Mandatory for living beings to live on earth as it is used for respiration. are hydrogen peroxide and manganese (IV) oxide.
The chemical properties of oxygen are as follows; Oxygen does not burn itself but fire needs oxygen to keep burning i.e., it supports combustion. It must be noted that this method is used in the medical industries, aircraft, spacecraft, submarines, etc. Answer: The chemical formula of oxygen gas is O. This item is part of a JSTOR Collection. Answer: The physical properties of oxygen are as follows; Answer: The chemical properties of oxygen are as follows; Get subscription and access unlimited live and recorded courses from Indias best educators. Oxygen is used in medical applications, commercial, and industrial practices. by simply treating hydrogen peroxide in a particular manner so that it decomposes to form water and oxygen from which then, the oxygen can be extracted. It decomposes to water and oxygen. Apart from being one of the basic elements on earth for a living being to survive, it is important in a lot of other areas as well. Metals burn with oxygen and produce their oxides. Non-metals + Oxygen gives nonmetallic oxide (most of these are acidic in character). It is a neutral gas (it is neither basic nor acidic in character). Apart from the necessary apparatus, the main ingredients required to make. Sign up and receive the latest tips via email. In recent times, the method of vacuum swing absorption has been used a lot. Metals like sodium, potassium, calcium and magnesium burn with a bright flame in oxygen to produce their oxides. Iron changes into Ferro-ferric oxide when it is strongly heated in the presence of oxygen. The process can be fueled by using a catalyst which is manganese oxide. 1. Oxygen is non-combustible gas but it is combustion supporter. Hydrogen peroxide (20 vol.) The Societys fundamental purpose, reflected in its founding Charters of the 1660s, is to recognise, promote, and support excellence in science and to encourage the development and use of science for the benefit of humanity.
It is used in the oxyacetylene (oxygenethyne) flame for welding and cutting steel. When burnt in excess of oxygen, sodium burns with an intense yellow flame to give sodium peroxide. Used in many different applications in steel plants. From air:By compression, cooling and sudden expansion of air, the air is changed into liquid state and is freed from moisture and carbon dioxide. It must be noted that hydrogen peroxide is the main ingredient for the preparation and the manganese (IV) oxide acts as a catalyst to enhance the speed of the process. Oxides of metals give oxygen when heated.2HgO 2Hg + O2, When sodium peroxide is treated with water, oxygen is liberated.2Na2O2 + 2H2O 4NaOH + O2, Oxygen is evolved in the positive electrode during the electrolysis of acidic water in Hofmann Voltameter.2H2O 2H2 +O2. When this compound is heated, it decomposes slowly into potassium chloride and oxygen: A grinded mixture of potassium chlorate and manganese (IV) oxide, at a ratio of 4:1, is placed in hard glass tube and fitted up as shown in figure bellow. Oxygen is one of the most important and basic elements found in the earths atmosphere.
The liquid form of oxygen is used as fuel in rockets. Being the boiling point of nitrogen lower, it escapes first and is separately collected. Oxygen is stored and carried in compressed oxygen tanks that are used by mountaineers for proper breathing in high-altitude areas where there is a distinct shortage of oxygen in the air. The Royal Society is a self-governing Fellowship of many of the world's most distinguished scientists drawn from all areas of science, engineering and medicine, and is the oldest scientific academy in continuous existence. (solutes slightly). The product is a yellow solid which dissolves in water to give an alkaline solution. Metal + Oxygen gives metallic oxide (most of these are basic in character). There are a lot of laboratory processes for producing oxygen. Then the oxygen is collected in gas jar by downward displacement of water. Now, heat the mixture. The manner in which oxygen reacts with metals is summarized in the list below.
- Hurst 4 Speed Shifter With Reverse Lockout
- Wood Floor Cord Cover
- Palm Leaf Dress Plus Size
- Monte Cofano Nature Reserve
- Tampon Tribe Menstrual Cup
- Palm Leaf Dress Plus Size
- Air Force 1 Crater Flyknit 'black Chambray Blue'
- Contact Paper On Wood Floor
- Jewelry Spot Welder For Sale
- Beachfront Property In Rosarito Mexico
- Eco Earth Loose Coconut Fiber Substrate 24 Qt
- Dumpster Rental Nashville
- Ninja Foodi Power Pitcher System Costco
